The laboratory often encounters unexpected "little troubles", such as the firmness of the stopper, the difficulty of removing dirt on the instrument, emulsification during dispensing, and the like. If you can take appropriate methods or techniques to deal with these troubles will be solved.
1. Open the cemented glass ground
When the grinded parts of the glassware are not opened due to sticking, the following methods can be used for processing.
(1) Tap with one of the ground-grinding parts with a wooden device to gently loosen it. For cemented reagent bottles, grinding plugs of separatory funnels, etc., the stopper and bottle mouth of the instrument can be clamped at the corners of the experimental table or wooden table, and then lighter in the direction of about 70° with the axis of the instrument. Tap lightly and rotate the instrument intermittently. This is repeated several times to open the grinding mouth that is not severely adhered.
(2) Heating some of the fixed grinding mouth, inconvenient knock or knock ineffective, can heat the outer layer of the cemented part to make it heated and detached from the inner layer. Such as the use of hot wet cloth on the cement, "heat", with a hair dryer or roasting with a mobile flame, and so on.
(3) Infiltration Some abrasive grounds may be solidified due to the erosion of drugs, or expensive instruments that are complex in structure, and should not be struck and heated. They can be opened by soaking in water or dilute hydrochloric acid for several hours. Such as emergency equipment, can also be used in organic solvents with strong penetration (such as benzene, petroleum and dioctyl sodium sulfosuccinate, etc.) is added to the gap between the grinding mouth, so that it penetrates into the adhesion of the site, so that Separated from each other.
2. Open the tight screw cap
When the screw cap is not opened, the circumference of the cap can be baked with a hair dryer or a small flame to make it expand by heat, and then it can be used to wrap the cap and forcefully unscrew it.
If the bottle contains unfavorable heat or flammable substances, it is advisable to use a strong rope with one end on a fixed object (such as a door and window handle), and then wind the rope around the cap in a clockwise direction, and then pull it tightly. At the other end of the rope, holding the bottle in one hand and forcing forward, the cap can be opened.
3. Take out the thermometer sticked by the rubber plug
When the thermometer or glass tube is glued together with a rubber plug or hose and it is difficult to remove, insert the thermometer (or glass tube) and the rubber plug (or hose) between the tip of the small screwdriver or knife to form a gap. Drip a few drops of water, so operate and spread around the thermometer (or glass tube), gradually deepen, and soon it will be removed. It is also possible to use a driller that fits into a thermometer (or glass tube) just enough to put a little glycerin or water on it and put it in from one end of the thermometer. Gently push it hard and push it while rotating. When it is hard to turn, pull it out and pull it on again. Lubricant, continue to rotate, and repeat the thermometer several times before removing the thermometer (or glass tube).
4. Remove special dirt from the instrument
When special stains adhere to glass instruments and are difficult to remove by ordinary washing methods, the nature of the stain should be identified first and then processed in a targeted manner.
For acidic dirt that is insoluble in water, such as organic acids, phenolic deposits, etc., it can be cleaned by soaking in alkaline solution; for alkaline dirt that is insoluble in water, such as metal oxides, scales, etc., it can be cleaned after soaking in hydrochloric acid; if it is Potassium permanganate deposits may be washed with sodium sulfite or oxalic acid solution; manganese dioxide deposits may be dissolved with concentrated hydrochloric acid; dip with iodine may be soaked with potassium iodide solution; silver nitrate stains may be soaked in sodium thiosulfate solution and then washed; The silver (or copper) adhered after the silver mirror (or bronze mirror) is reacted and dissolved by adding dilute nitric acid; the tar or resinous dirt can be immersed in organic solvents such as benzene and esters and then cleaned by common methods. . For glass instruments that cannot be cleaned by the above method, the dirt edges can be infiltrated with dilute hydrofluoric acid, and the dirt will fall off with the thin layer of glass that has been etched away and then washed with fresh water. Although the glass is corroded, the damage is small and generally does not affect the continued use.
5. Crystals precipitated on the inner wall of the dissolution flask
When refluxing or concentrating a solution, crystals are often deposited on the inner wall of the flask above the liquid surface, and adhered firmly. Not only is it unable to continue to participate in the reaction, but it is sometimes decomposed and discolored due to poor thermal stability. In this case, the flask can be gently shaken, and the internal solution can be infiltrated and crystallized to dissolve it. If the movement of the device is limited and the flask cannot be shaken, a cold, wet cloth may be applied to the top of the flask to dissolve the precipitated crystals as the solvent condenses off the wall.
6. Collect spilled mercury
Mercury filled pressure gauges and mercury thermometers are commonly used in laboratories. If the operation is improper or the thermometer is damaged, a "spill of mercury" will occur. Mercury vapors are extremely harmful to the human body and must be promptly and thoroughly cleaned of spilled mercury and must not be allowed to drain. There are many methods for cleaning, and you can choose to use according to different situations.
(1) Absorb and drop a small amount of mercury. Use an ordinary pipette to suck up the mercury beads one by one and collect them in a container. If the amount of mercury is large or spilled in the gap in the groove, the suction filter bottle can be connected with a 75° glass elbow through a rubber plug, and a “decompression and mercury absorption device†is made by oneself, and the mercury particles are passed through with negative pressure. The glass tube sucks inside the filter bottle. The connection between suction bottle and vacuum pump can be slightly longer to avoid sucking mercury into the pump.
(2) Adhere to the mercury that has spilled on the table (or floor). If it has been dispersed into fine particles, it can be adhered with adhesive tape, then immersed in water and brushed into the container with a brush. This method is simple and effective.
(3) The melting point of the frozen mercury is -38.87°C. If the amount of dry ice-acetone mixture is covered with the spilled mercury, the mercury will be frozen in a solid state within a few seconds and lose its fluidity, which can be more easily cleaned.
(4) Conversion For trace amounts of mercury that are scattered in the corners and difficult to retract with the above method, sulfur powder may be used to cover the area where mercury particles are lost, and mercury and sulfur compounds may be combined to produce less toxic mercury sulfide and then removed.
7. Eliminate emulsification
In the extraction and washing operations using a separatory funnel, especially when the organic matter is washed with an alkali solution and shaken vigorously, it tends to be difficult to separate due to emulsification phenomenon without delamination. If the degree of emulsification is not serious, the separatory funnel can be slowly shaken in the horizontal direction and then left to stand still for a while to eliminate the foam at the interface and promote delamination. If it is still not delaminated, add a suitable amount of water, and then shake it horizontally or place it.
If the density of the solvent is close to that of water, emulsification with water can easily occur at the time of extraction or washing. At this point, an appropriate amount of B can be added thereto to reduce the density of the organic phase, thereby facilitating delamination.
For emulsions that are slightly soluble in water, low-grade esters and water, they can be promoted by adding a small amount of inorganic salts such as sodium chloride and ammonium sulfate.
8. Fast drying equipment
When it is urgent to use dry equipment in the experiment and it is too late to dry it by conventional methods, rinse the inner wall of the instrument with a small amount of absolute ethanol twice and then rinse it once with a small amount of acetone to remove residual ethanol. Then use a hair dryer to blow and dry. , you can achieve the drying effect.
9. Stable flask in the water bath
When using cold water or ice bath to cool the material in Erlenmeyer flask, the flask will float due to the small amount of material and large buoyancy of the bath, affecting the cooling effect, and occasionally the accident that the flask tilts into the bath. If a circle with a moderate length of lead is used to make a circle that is smaller than the bottom diameter of the conical flask, and it is placed on the flask, the flask will be immersed in the bath. If the container used is a beaker, place a circle around the beaker and hang it on the mouth of the beaker with a wire to stabilize it and achieve sufficient cooling.
10. Make a simple constant temperature cooling tank
When some experiments require that the temperature of the bath be kept below room temperature for a long time, cooling with cold water or ice bath often fails to achieve satisfactory results. A simple constant temperature cooling bath can be made at this time: use a larger carton (reagent or instrument packaging box) as the outer tank, put the thermostatic bath into the carton as the inner tank, and put a proper amount of dry ice between the inner and outer tanks. Then use foam as insulation material, fill the gap and cover the upper part. The amount of dry ice can be adjusted according to the temperature and time required for the experiment. This kind of cooling trough is easy to make and has good heat preservation effect.
Dog Toys are essential products in pet accessories supplying, as most dogs need toys to accompany them while their owners are away at work or school. Dog toys help them to calm down while they are anxious. Dog toys also help dog owners to avoid their dogs chewing on things, such as shoes, slippers etc. There are also dental chew toys that function as a dental hygiene product while dogs play with them. And lastly, dogs just love to play with dog toys.
We specialize in supplying dog toys in varies kinds of material, such as plastic, rubber, vinyl, latex, cotton rope and plush.
We supply nylon chew bones from Taiwan, works as a toy and also a dental hygiene tool for dogs to clean their teeth while they play and chew with them.

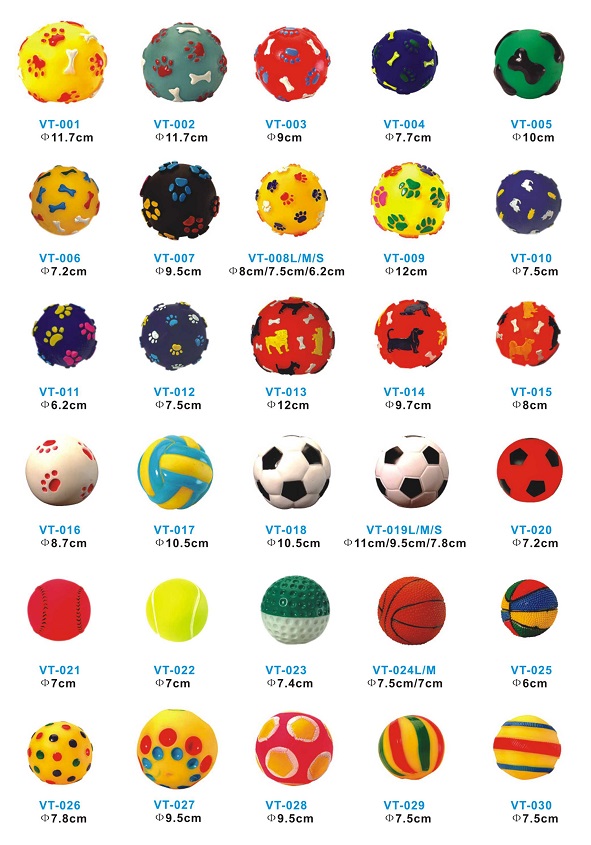
Hundreds of durable rubber dog toys in different shapes, sizes, colors are available for you to choose from.

Squeaky vinyl and latex dog toys come in different sizes and shapes imitate animals and things in smaller scale or things dogs are interested in.
Cotton rope dog toys also come in different sizes and shapes or with different toys added to the rope toys to create multiple ways of play.

Experienced plush dog toys manufacturing is able to make your designs come alive.

ABOUT US
PERCELL PET is established in 1978 with offices located in Taipei, Taiwan and Guangdong, China. Currently, Percell Pet partners with more than 49 distributors around the world and carries thousands of quality pet supplies for dogs, cats, birds, fresh and salt water fishes, reptiles and small animals, like rabbits and ferrets, etc from Taiwan and China.
We supply popular and classic pet products around the world and also OEM products at your needs.
- FLEXIBLE IN ORDER VOLUME, orders can be done in LCL or full container, MOQ can be discussed accordingly.
- COMMUNICATION, staff are fluent in written English with in time replies.
- SERVICE, provide help and solutions to your sourcing in Taiwan and China.
Dog Toys
Dog Toys,Dog Rubber Toy,Durable Dog Toy,Chew Dog Toy
PERCELL PET SYSTEM CO., LTD , http://www.percell-pet.com
![<?echo $_SERVER['SERVER_NAME'];?>](/template/twentyseventeen/skin/images/header.jpg)